UNDER CONSTRUCTION
The grey-headed flying-fox, Pteropus poliocephalus
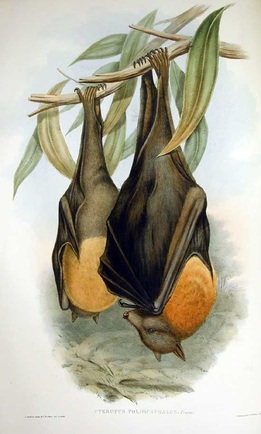
The grey-headed flying-fox, Pteropus poliocephalus (Figure 1) is of the order Chiroptera, suborder Megachiroptera, family Pteropodidae, subfamily Pteropodinae, and genus Pteropus. The species was first described in 1825 by Temminck.
General characteristics
P. poliocephalus is dark brown except for an orange/brown mantle that fully encircles the neck and its fur extends right down the legs to the toes (Hall & Richards, 2000). Adult individuals weigh between 600-1000 g with a maximum-recorded weight of 1070 g and have a wingspan exceeding one 1.5 metres (Welbergen, 2005). P. poliocephalus is long-lived and there are reports of individuals surviving in captivity for up to 23 years (Pritchard, 2001) and a maximum age of up to 15 years seems possible in the wild (Tidemann, 1999).
Behaviour
Roosting
Like most Pteropus species, P. poliocephalus forms colonies (i.e. “camps” or “day roosts”) during the day on the exposed branches, commonly of canopy trees (Figure 2) (Eby, 1996; Mickleburgh et al., 1992; Nelson, 1963, 1965a, b; Ratcliffe, 1931, 1932; Tidemann et al., 1999). P. poliocephalus colonies may contain thousands of individuals (Nelson, 1965b; Tidemann et al., 1999). Ratcliffe (1931) reported colony size estimates of over 200,000 in many colony sites and such camps were found into the 1990’s (Eby, 1991). Recent counts however have found few colonies over 20,000 individuals (Eby et al., 1999).
Colonies are formed in seemingly arbitrary locations (Tidemann et al., 1999). Roost vegetation includes rainforest patches, stands of Melaleuca, mangroves and riparian vegetation (Nelson, 1965b; Ratcliffe, 1931), but colonies also use highly modified vegetation in urban areas (e.g. Birt & Markus, 1999; Tidemann & Vardon, 1997). P. poliocephalus exhibits a high seasonal fidelity to traditional colonies and return annually to the same locations (Augee & Ford, 1999; Lunney & Moon, 1997; Nelson, 1963). When undisturbed, colony locations can be stable for several decades and fidelity to some sites may even have pre-dated human settlement (Augee & Ford, 1999; Lunney & Moon, 1997; Nelson, 1963). In these colonies, mating, parturition and rearing of the young takes place.
An important determinant of colony size is food available within nightly foraging distances, commonly within 20 kilometres but up to 50 km (Eby, 1996; Parry-Jones & Augee, 1991; Parry-Jones & Augee, 2001). Another important determinant is the time of year with large aggregations of individuals occurring mainly during the mating seasons (Nelson, 1965b; Parry-Jones & Augee, 2001).
Like most Pteropus species, P. poliocephalus forms colonies (i.e. “camps” or “day roosts”) during the day on the exposed branches, commonly of canopy trees (Figure 2) (Eby, 1996; Mickleburgh et al., 1992; Nelson, 1963, 1965a, b; Ratcliffe, 1931, 1932; Tidemann et al., 1999). P. poliocephalus colonies may contain thousands of individuals (Nelson, 1965b; Tidemann et al., 1999). Ratcliffe (1931) reported colony size estimates of over 200,000 in many colony sites and such camps were found into the 1990’s (Eby, 1991). Recent counts however have found few colonies over 20,000 individuals (Eby et al., 1999).
Colonies are formed in seemingly arbitrary locations (Tidemann et al., 1999). Roost vegetation includes rainforest patches, stands of Melaleuca, mangroves and riparian vegetation (Nelson, 1965b; Ratcliffe, 1931), but colonies also use highly modified vegetation in urban areas (e.g. Birt & Markus, 1999; Tidemann & Vardon, 1997). P. poliocephalus exhibits a high seasonal fidelity to traditional colonies and return annually to the same locations (Augee & Ford, 1999; Lunney & Moon, 1997; Nelson, 1963). When undisturbed, colony locations can be stable for several decades and fidelity to some sites may even have pre-dated human settlement (Augee & Ford, 1999; Lunney & Moon, 1997; Nelson, 1963). In these colonies, mating, parturition and rearing of the young takes place.
An important determinant of colony size is food available within nightly foraging distances, commonly within 20 kilometres but up to 50 km (Eby, 1996; Parry-Jones & Augee, 1991; Parry-Jones & Augee, 2001). Another important determinant is the time of year with large aggregations of individuals occurring mainly during the mating seasons (Nelson, 1965b; Parry-Jones & Augee, 2001).
Foraging
At dusk the grey-headed flying-fox emerges from the colony to forage for floral resources (i.e. blossom, pollen, nectar and fruit) (Eby, 1991, 1995, 1996; Fujita & Tuttle, 1991; Spencer et al., 1991). The exact timing of the start of the emergence reflects the outcome of a trade-off between the risk of predation and the need for foraging (Welbergen, 2005). The emergence timing of males also depends on their social status: bachelor males leave before harem holders and the latter leave only when the last female in their harem has left.
The food sources usually associated with P. poliocephalus belong mainly to the genera Eucalyptus,Angophora, Melaleuca and Banksia, but in some areas it also utilises a wide range of rainforest fruit (Eby, 1998; Parry-Jones, 1987; Parry-Jones & Augee, 1991; Ratcliffe, 1932). P. poliocephalus is the only mammalian frugivore to occupy substantial areas of subtropical rainforests and so is of key importance for seed dispersal in those forests (Eby, 1996; Mickleburgh et al., 1992). Most vegetation communities used byP. poliocephalus produce foraging resources in seasonal but annually irregular superabundant pulses, andP. poliocephalus has adopted complex migration traits in response to such ephemeral and patchy food resources (Eby, 1996, 1998; Nelson, 1965b; Parry-Jones & Augee, 1992; Spencer et al., 1991). However, there are some temporally and spatially reliable resources restricted to a small number of coastal vegetation communities in northern New South Wales and Queensland that may support smaller resident populations (Eby, 1996). Cultivated orchard fruits are also taken but apparently only at times when other food items are scarce (Parry-Jones & Augee, 1991).
At dusk the grey-headed flying-fox emerges from the colony to forage for floral resources (i.e. blossom, pollen, nectar and fruit) (Eby, 1991, 1995, 1996; Fujita & Tuttle, 1991; Spencer et al., 1991). The exact timing of the start of the emergence reflects the outcome of a trade-off between the risk of predation and the need for foraging (Welbergen, 2005). The emergence timing of males also depends on their social status: bachelor males leave before harem holders and the latter leave only when the last female in their harem has left.
The food sources usually associated with P. poliocephalus belong mainly to the genera Eucalyptus,Angophora, Melaleuca and Banksia, but in some areas it also utilises a wide range of rainforest fruit (Eby, 1998; Parry-Jones, 1987; Parry-Jones & Augee, 1991; Ratcliffe, 1932). P. poliocephalus is the only mammalian frugivore to occupy substantial areas of subtropical rainforests and so is of key importance for seed dispersal in those forests (Eby, 1996; Mickleburgh et al., 1992). Most vegetation communities used byP. poliocephalus produce foraging resources in seasonal but annually irregular superabundant pulses, andP. poliocephalus has adopted complex migration traits in response to such ephemeral and patchy food resources (Eby, 1996, 1998; Nelson, 1965b; Parry-Jones & Augee, 1992; Spencer et al., 1991). However, there are some temporally and spatially reliable resources restricted to a small number of coastal vegetation communities in northern New South Wales and Queensland that may support smaller resident populations (Eby, 1996). Cultivated orchard fruits are also taken but apparently only at times when other food items are scarce (Parry-Jones & Augee, 1991).
Territoriality
From January onwards males set up ‘mating territories’ in the colony that are actively maintained by aggression and scent-marking (Figure 3) (Nelson, 1965a; Welbergen, 2005). The average territory size is about 3.5 body lengths along branches (Welbergen, 2005). The neck of P. poliocephalus contains androgen-sensitive sebaceous neck glands which are enlarged in breeding-season males (Martin et al., 1995) and are used frequently for scent-marking the entire length of the branches within their territories (Welbergen, 2005).
In the colonies territorial interactions (i.e., chases, biting, beating with wings) were always between resident and intruding males, never between females (Welbergen, 2005; contrary to Nelson, 1965a). During the mating season (April-May: Martin et al., 1993; Martin et al., 1995; Martin et al., 1987) territory defence is especially evident (Nelson, 1965a) when male mating territories may contain up to five females. During this time fights can involve up to 10 individuals fighting simultaneously (J. Welbergen, pers. obs.).
Intruding males fly around in circles of approximately 20 m diameter and attempt to crash-land onto the territory holder or the territory that it is guarding. This behaviour is remarkably similar to the intruder behaviour (sensu Richards, 1995) of P. conspicillatus that I also observed in P. poliocephalus at the feeding sites.
Males are also territorial in the feeding areas (J. Welbergen, pers. obs.); however, this territorial behaviour does not seem to function to obtain matings as matings at the feeding sites are extremely rare (see below). In fact, during the mating season at night, males are mostly separated from females because the majority of males return to the colony approximately two hours after they have started foraging (Welbergen, 2005). The majority of behaviours that are displayed by the males at night in the colony involve scent-marking, territorial fighting and the uttering of territorial cries (sensu Nelson, 1964) (Welbergen, 2005). Only around dawn, several hours later, are these males rejoined by females (Welbergen, 2005).
From January onwards males set up ‘mating territories’ in the colony that are actively maintained by aggression and scent-marking (Figure 3) (Nelson, 1965a; Welbergen, 2005). The average territory size is about 3.5 body lengths along branches (Welbergen, 2005). The neck of P. poliocephalus contains androgen-sensitive sebaceous neck glands which are enlarged in breeding-season males (Martin et al., 1995) and are used frequently for scent-marking the entire length of the branches within their territories (Welbergen, 2005).
In the colonies territorial interactions (i.e., chases, biting, beating with wings) were always between resident and intruding males, never between females (Welbergen, 2005; contrary to Nelson, 1965a). During the mating season (April-May: Martin et al., 1993; Martin et al., 1995; Martin et al., 1987) territory defence is especially evident (Nelson, 1965a) when male mating territories may contain up to five females. During this time fights can involve up to 10 individuals fighting simultaneously (J. Welbergen, pers. obs.).
Intruding males fly around in circles of approximately 20 m diameter and attempt to crash-land onto the territory holder or the territory that it is guarding. This behaviour is remarkably similar to the intruder behaviour (sensu Richards, 1995) of P. conspicillatus that I also observed in P. poliocephalus at the feeding sites.
Males are also territorial in the feeding areas (J. Welbergen, pers. obs.); however, this territorial behaviour does not seem to function to obtain matings as matings at the feeding sites are extremely rare (see below). In fact, during the mating season at night, males are mostly separated from females because the majority of males return to the colony approximately two hours after they have started foraging (Welbergen, 2005). The majority of behaviours that are displayed by the males at night in the colony involve scent-marking, territorial fighting and the uttering of territorial cries (sensu Nelson, 1964) (Welbergen, 2005). Only around dawn, several hours later, are these males rejoined by females (Welbergen, 2005).
Mating
Matings are generally observed between March and May (Martin et al., 1993; Martin et al., 1995; Martin et al., 1987; Nelson, 1965a) but the most likely time of conception is April (Martin et al., 1993; Martin et al., 1995; Martin et al., 1987) when there is a concomitant peak in mating activity (Welbergen, 2005). Mating is almost entirely restricted to the mating territories in the colonies during the day; mating at night, outside the context of the mating territories, is extremely rare as it has only been observed once (J. Welbergen, pers. obs.) despite many hours of observations on individual P. poliocephalus in the feeding areas by others and myself (e.g. Eby, 1996; McWilliam, 1986; Nelson, 1965a). Mating is a highly stereo-typed and energetically demanding behaviour (Welbergen, 2001; see also Markus, 2002 for mating behaviour in the closely relatedP. alecto). Females have an active role in the initiation and termination of copulations (Welbergen, 2002). Males mate repeatedly with the same female during a mating session that can last up to one hour, and have multiple mating sessions with the same female over the course of several days (Welbergen, 2002).
Unambiguous extra-group copulations have been recorded (J. Welbergen, pers. obs.); in all cases it was a neighbouring male that mated with a female of a harem the holder of which had left due to a disturbance (see also Kaiser, 2004). Matings occurred all through the day but increased in frequency during sudden bouts of mass-mating (J. Welbergen, pers. obs.) similar to what has been observed in P. giganteus (Neuweiler, 1968).
Matings are generally observed between March and May (Martin et al., 1993; Martin et al., 1995; Martin et al., 1987; Nelson, 1965a) but the most likely time of conception is April (Martin et al., 1993; Martin et al., 1995; Martin et al., 1987) when there is a concomitant peak in mating activity (Welbergen, 2005). Mating is almost entirely restricted to the mating territories in the colonies during the day; mating at night, outside the context of the mating territories, is extremely rare as it has only been observed once (J. Welbergen, pers. obs.) despite many hours of observations on individual P. poliocephalus in the feeding areas by others and myself (e.g. Eby, 1996; McWilliam, 1986; Nelson, 1965a). Mating is a highly stereo-typed and energetically demanding behaviour (Welbergen, 2001; see also Markus, 2002 for mating behaviour in the closely relatedP. alecto). Females have an active role in the initiation and termination of copulations (Welbergen, 2002). Males mate repeatedly with the same female during a mating session that can last up to one hour, and have multiple mating sessions with the same female over the course of several days (Welbergen, 2002).
Unambiguous extra-group copulations have been recorded (J. Welbergen, pers. obs.); in all cases it was a neighbouring male that mated with a female of a harem the holder of which had left due to a disturbance (see also Kaiser, 2004). Matings occurred all through the day but increased in frequency during sudden bouts of mass-mating (J. Welbergen, pers. obs.) similar to what has been observed in P. giganteus (Neuweiler, 1968).
Gestation and parturition
P. poliocephalus breeds seasonally and the majority of females reproduce once a year when they give birth to a single young (Eby, 1995; Hall & Richards, 2000). Parturition usually occurs in October (Eby, 1995; Nelson, 1965a) after a gestation period of about 27 weeks (Martin et al., 1987; O'Brien, 1993). The young are born altrical and are dependent on their mothers for temperature regulation (Bartholomew et al., 1964). Females carry their young for the first three weeks during their nightly foraging trips and after this time young are left in the colony at night (Eby, 1995). Young are capable of independent flight in January at which time they commence foraging outside the colony (Eby, 1995). Young are weaned between February-April (Eby, 1995; Martin et al., 1995; Nelson, 1965a; Pook, 1977; Welbergen, 2002).
P. poliocephalus breeds seasonally and the majority of females reproduce once a year when they give birth to a single young (Eby, 1995; Hall & Richards, 2000). Parturition usually occurs in October (Eby, 1995; Nelson, 1965a) after a gestation period of about 27 weeks (Martin et al., 1987; O'Brien, 1993). The young are born altrical and are dependent on their mothers for temperature regulation (Bartholomew et al., 1964). Females carry their young for the first three weeks during their nightly foraging trips and after this time young are left in the colony at night (Eby, 1995). Young are capable of independent flight in January at which time they commence foraging outside the colony (Eby, 1995). Young are weaned between February-April (Eby, 1995; Martin et al., 1995; Nelson, 1965a; Pook, 1977; Welbergen, 2002).
Early observations on social organisation of P. poliocephalus
Variability in mating systems is a consequence of variability in the characteristics of female dispersion (e.g. Clutton-Brock, 1989) and early observations (i.e. Nelson, 1965a) suggested that in P. poliocephalus female dispersion varies both on a spatial and temporal scale and social organisation is complex.
The spatial variability in female dispersion can be found within the colony: Nelson (1965a) reported that harems (Figure 4) were larger in the higher-density centres of colonies.
Males with territories in the centre of the colonies were polygynous whereas males with territories away from the centre were monogamous. Males roosting near the periphery of the colonies rarely roosted with females and conceivably engaged in some form of non-parental polyandry. Nelson (1965a) also noted a higher degree of monogamy among males that form mixed-sex groups with females that were still nursing their young from the previous year. He further reported that males set up territories around females suggesting some form of female defence strategy. Female dispersion varied temporally between the colony during the day and the feeding sites at night. At the feeding sites, males also defended feeding territories (Nelson, 1965a) suggesting that some form of resource defence strategy. Based on the above information, the mating system of P. poliocephalus proved difficult to categorise according to the scheme used by McCracken and Wilkinson (2000). For convenience they categorised P. poliocephalus as mating in multi-male/multi-female polygynous groups.
Bradbury suggested four criteria to distinguish leks from other mating systems (Bradbury, 1981; Bradbury & Gibson, 1983): (1) males aggregate at specific sites for display; (2) females can select their mate(s); (3) there is no male parental care so that males contribute nothing to the next generation apart from gametes; (4) the only resource females find at these sites are the males themselves.
The mating system of P. poliocephalus seems to fulfil the first three of these criteria (Welbergen, 2005): (1) males held mating territories that are costly to maintain and the highest quality males had the most central mating territories; (2) from observing general spatial shifts in the composition of the primary study colony and from observing the changes in the harem composition of individually marked males, it became clear that towards the start of the mating season females moved from the colony’s periphery into the centre of the colony and into the mating territories of males forming harems consisting of unstable female groups; (3) males do not provide any parental care as they never roost next to their offspring and any adult male – young interactions that could be construed as such were never observed. Mating territories do provide a place for females to roost and, strictly speaking, therefore criterion (4) is not satisfied. However, since roosting space was found not to be limiting in this species, the only essential resource in a mating territory seems the male itself.
Since mating on leks is never random and reproductive success tends to be skewed towards a limited number of males, it is of interest to know what makes some males more attractive than others. Possible cues for female preferences are display execution (including advertisement of intrinsic male traits such as health, age or size) and/or locale within the lek. The latter is cited as a major determinant of mate choice on leks (e.g. Borgia, 1979; Emlen & Oring, 1977; Kokko et al., 1999). Since in P. poliocephalus competition for the central territories seems more severe in the more central territories (Kaiser, 2004), males are expected to become aligned spatially in order of increasing competitive ability towards the centre of the colony. Indeed, in P. poliocephalus: male characteristics that commonly covary with competitive ability such as body size and body weight increased towards the centre of the colony (Welbergen, 2005). Thus it is possible that in P. poliocephalus, males use the location of a male’s territory as an indication of his quality. This would make the task of finding a suitable mate in a colony that contains tens of thousands of individuals much more efficient as it would enable a female to quickly discard a large number of low-quality males. Thus, the locale within the lek becomes the male’s ‘display execution’ and therefore part of the male’s sexually selected extended phenotype.
The spatial variability in female dispersion can be found within the colony: Nelson (1965a) reported that harems (Figure 4) were larger in the higher-density centres of colonies.
Males with territories in the centre of the colonies were polygynous whereas males with territories away from the centre were monogamous. Males roosting near the periphery of the colonies rarely roosted with females and conceivably engaged in some form of non-parental polyandry. Nelson (1965a) also noted a higher degree of monogamy among males that form mixed-sex groups with females that were still nursing their young from the previous year. He further reported that males set up territories around females suggesting some form of female defence strategy. Female dispersion varied temporally between the colony during the day and the feeding sites at night. At the feeding sites, males also defended feeding territories (Nelson, 1965a) suggesting that some form of resource defence strategy. Based on the above information, the mating system of P. poliocephalus proved difficult to categorise according to the scheme used by McCracken and Wilkinson (2000). For convenience they categorised P. poliocephalus as mating in multi-male/multi-female polygynous groups.
Bradbury suggested four criteria to distinguish leks from other mating systems (Bradbury, 1981; Bradbury & Gibson, 1983): (1) males aggregate at specific sites for display; (2) females can select their mate(s); (3) there is no male parental care so that males contribute nothing to the next generation apart from gametes; (4) the only resource females find at these sites are the males themselves.
The mating system of P. poliocephalus seems to fulfil the first three of these criteria (Welbergen, 2005): (1) males held mating territories that are costly to maintain and the highest quality males had the most central mating territories; (2) from observing general spatial shifts in the composition of the primary study colony and from observing the changes in the harem composition of individually marked males, it became clear that towards the start of the mating season females moved from the colony’s periphery into the centre of the colony and into the mating territories of males forming harems consisting of unstable female groups; (3) males do not provide any parental care as they never roost next to their offspring and any adult male – young interactions that could be construed as such were never observed. Mating territories do provide a place for females to roost and, strictly speaking, therefore criterion (4) is not satisfied. However, since roosting space was found not to be limiting in this species, the only essential resource in a mating territory seems the male itself.
Since mating on leks is never random and reproductive success tends to be skewed towards a limited number of males, it is of interest to know what makes some males more attractive than others. Possible cues for female preferences are display execution (including advertisement of intrinsic male traits such as health, age or size) and/or locale within the lek. The latter is cited as a major determinant of mate choice on leks (e.g. Borgia, 1979; Emlen & Oring, 1977; Kokko et al., 1999). Since in P. poliocephalus competition for the central territories seems more severe in the more central territories (Kaiser, 2004), males are expected to become aligned spatially in order of increasing competitive ability towards the centre of the colony. Indeed, in P. poliocephalus: male characteristics that commonly covary with competitive ability such as body size and body weight increased towards the centre of the colony (Welbergen, 2005). Thus it is possible that in P. poliocephalus, males use the location of a male’s territory as an indication of his quality. This would make the task of finding a suitable mate in a colony that contains tens of thousands of individuals much more efficient as it would enable a female to quickly discard a large number of low-quality males. Thus, the locale within the lek becomes the male’s ‘display execution’ and therefore part of the male’s sexually selected extended phenotype.
Conservation & Threats
P. poliocephalus was once considered to be an abundant species with numbers estimated in the many millions (Ratcliffe, 1931).
Currently, the species is exposed to several threatening processes, the most serious of which is loss of foraging and roosting habitat (e.g. Eby, 1995; Tidemann, 1999). European settlement, initiated in 1788, engendered extreme habitat loss and fragmentation across most of the P. poliocephalus range (Tidemann, 1999), with diverse and mostly detrimental impacts on biodiversity (Saunders et al., 1990).
Another serious threat is direct killing of animals in orchards and harassment and destruction of roosts. P. poliocephalus destroys commercial fruit in Queensland and New South Wales (Tidemann & Vardon, 1997). As a consequence P. poliocephalus is regarded as a pest species over much of its range (e.g. Hall, 1987). In the past decade negative public perception of the species has intensified with the discovery of three new diseases that are potentially fatal to humans, Hendravirus, Menanglevirus and Australian Bat Lyssavirus (Allworth et al., 1996; Fraser et al., 1996; Halpin et al., 1999), so persecution has further intensified (Tidemann et al., 1999). The exact number of animals destroyed is unknown, but estimates as high as 100,000 annually have been made (Vardon & Tidemann, 1995). The impact is more substantial than direct deaths alone would indicate, for a large proportion of animals shot on orchards are pregnant and lactating females (Tidemann et al., 1997).
Competition with P. alecto (and perhaps P. scapulatus) may also be a threat to P. poliocephalus. The distribution of P. alecto has undergone a substantial southerly shift since 75 years ago (Eby & Palmer, 1991; Nelson, 1965b; Ratcliffe, 1932; Welbergen, 2002; personal observations) extending further into coastal areas inhabited by P. poliocephalus (Webb & Tidemann, 1995). The two species share roosts and diet plants, and there is tentative evidence that under conditions of increased habitat loss, the southwards expanding P. alecto becomes more competitive than P. poliocephalus in obtaining the remaining resources (Eby et al., 1999; Tidemann, 1999; Webb & Tidemann, 1995), although it has not been directly assessed. Furthermore, hybridisation seems possible (Webb & Tidemann, 1995) and I have observed several inter-specific matings between male P. alecto and female P. poliocephalus in the wild.
Temperature extremes have caused the death of tens of thousands of Australian flying-foxes in the last decade alone causing some of the most dramatic mass die-offs ever to have been recorded in mammals (Welbergen, 2005). Such extremes selectively affect the effective breeding population and recruitment of the species, which further exacerbates their impact (Welbergen, 2005). Since temperature extremes are expected to increase in the future (Meehl & Tebaldi, 2004), mass die-offs will become more frequent and widespread, and will occur at lower latitudes than previously. This will undoubtedly increase the threat to the survival of the species in addition to the anthropogenic factors that have already been identified.
Ratcliffe (1931), on the basis of anecdotal evidence, already believed that the species had declined by 50% since pre-European times as a result of clearing of native vegetation and competition with P. alecto. In recent years however, direct evidence has been accumulating that the species is indeed in serious decline (Eby et al., 1999; Hall & Richards, 2000). Current estimates for the species are about 400,000-700.000 and it has been suggested that the national population may have declined by as much as 30% in the last decade alone (Richards, 2000).
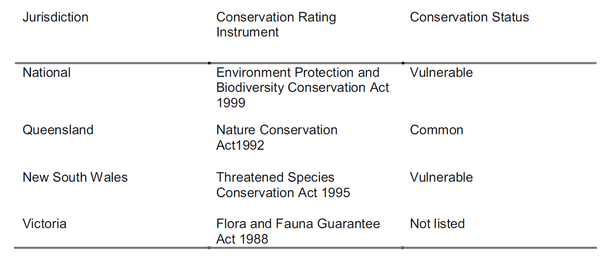
To answer these growing threats, roost sites have been legally protected since 1986 in New South Wales and 1994 in Queensland (Tidemann & Vardon, 1997), and in 1999 the species was classified as vulnerable to extinction in the Action Plan for Australian Bats (Duncan et al., 1999). The species is now protected both at state as well as federal level in Australia (Duncan et al., 1999; Eby et al., 1999; Tidemann, 2003) (Table 1), and as of 2008 the species has been listed as 'Vulnerable' on the IUCN Red List of Threatened Species (Lunney et al. 2008).
Currently, the species is exposed to several threatening processes, the most serious of which is loss of foraging and roosting habitat (e.g. Eby, 1995; Tidemann, 1999). European settlement, initiated in 1788, engendered extreme habitat loss and fragmentation across most of the P. poliocephalus range (Tidemann, 1999), with diverse and mostly detrimental impacts on biodiversity (Saunders et al., 1990).
Another serious threat is direct killing of animals in orchards and harassment and destruction of roosts. P. poliocephalus destroys commercial fruit in Queensland and New South Wales (Tidemann & Vardon, 1997). As a consequence P. poliocephalus is regarded as a pest species over much of its range (e.g. Hall, 1987). In the past decade negative public perception of the species has intensified with the discovery of three new diseases that are potentially fatal to humans, Hendravirus, Menanglevirus and Australian Bat Lyssavirus (Allworth et al., 1996; Fraser et al., 1996; Halpin et al., 1999), so persecution has further intensified (Tidemann et al., 1999). The exact number of animals destroyed is unknown, but estimates as high as 100,000 annually have been made (Vardon & Tidemann, 1995). The impact is more substantial than direct deaths alone would indicate, for a large proportion of animals shot on orchards are pregnant and lactating females (Tidemann et al., 1997).
Competition with P. alecto (and perhaps P. scapulatus) may also be a threat to P. poliocephalus. The distribution of P. alecto has undergone a substantial southerly shift since 75 years ago (Eby & Palmer, 1991; Nelson, 1965b; Ratcliffe, 1932; Welbergen, 2002; personal observations) extending further into coastal areas inhabited by P. poliocephalus (Webb & Tidemann, 1995). The two species share roosts and diet plants, and there is tentative evidence that under conditions of increased habitat loss, the southwards expanding P. alecto becomes more competitive than P. poliocephalus in obtaining the remaining resources (Eby et al., 1999; Tidemann, 1999; Webb & Tidemann, 1995), although it has not been directly assessed. Furthermore, hybridisation seems possible (Webb & Tidemann, 1995) and I have observed several inter-specific matings between male P. alecto and female P. poliocephalus in the wild.
Temperature extremes have caused the death of tens of thousands of Australian flying-foxes in the last decade alone causing some of the most dramatic mass die-offs ever to have been recorded in mammals (Welbergen, 2005). Such extremes selectively affect the effective breeding population and recruitment of the species, which further exacerbates their impact (Welbergen, 2005). Since temperature extremes are expected to increase in the future (Meehl & Tebaldi, 2004), mass die-offs will become more frequent and widespread, and will occur at lower latitudes than previously. This will undoubtedly increase the threat to the survival of the species in addition to the anthropogenic factors that have already been identified.
Ratcliffe (1931), on the basis of anecdotal evidence, already believed that the species had declined by 50% since pre-European times as a result of clearing of native vegetation and competition with P. alecto. In recent years however, direct evidence has been accumulating that the species is indeed in serious decline (Eby et al., 1999; Hall & Richards, 2000). Current estimates for the species are about 400,000-700.000 and it has been suggested that the national population may have declined by as much as 30% in the last decade alone (Richards, 2000).
To answer these growing threats, roost sites have been legally protected since 1986 in New South Wales and 1994 in Queensland (Tidemann & Vardon, 1997), and in 1999 the species was classified as vulnerable to extinction in the Action Plan for Australian Bats (Duncan et al., 1999). The species is now protected both at state as well as federal level in Australia (Duncan et al., 1999; Eby et al., 1999; Tidemann, 2003) (Table 1), and as of 2008 the species has been listed as 'Vulnerable' on the IUCN Red List of Threatened Species (Lunney et al. 2008).
The IUCN Action Plan for Old World Fruit Bats (Mickleburgh et al., 1992) and the Action Plan for Australian Bats (Duncan et al., 1999) lists lack of knowledge of the biology of Pteropus spp. as one of the main threats to their survival. The lack of information on the behaviour in colonies and at feeding sites severely limits our understanding of P. poliocephalus, and this understanding is particularly important now that it has become a prominent federal conservation problem in Australia.
References
- Allworth, A., Murray, K., & Morgan, J.A. (1996) human case of encephalitis due to a lyssavirus recently identified in fruit bats. Communicable Diseases Intelligence, 20, 504.
- Augee, M.L. & Ford, D. (1999) Radio-tracking studies of Grey-headed Flying-foxes, Pteropus poliocephalus, from the Gordon colony, Sydney. Proceedings of the Linnean Society of New South Wales, 121, 61-70.
- Bartholomew, G.A., Leitner, P., & Nelson, J.E. (1964) Body temperature oxygen consumption and heart rate in three species of Australian flying foxes. Physiological Zoology, 37, 179-198.
- Birt, P. & Markus, N. (1999) Notes on the temporary displacement of Pteropus alecto and P. poliocephalusby P. scapulatus within a daytime campsite. Australian Mammalogy, 21, 107-110.
- Borgia, G. (1979) Sexual selection and the evolution of mating systems. In Sexual selection and reproductive competition in insects. (eds M.S. Blum & N.A. Blum), pp. 19–80. Academic Press, . New York.
- Bradbury, J.W. (1981) The evolution of leks. In Natural selection and social behavior. (eds R.D. Alexander & D.W. Tinkle), pp. 138-169. Chiron Press, New York.
- Bradbury, J.W. & Gibson, R.M. (1983) Leks and mate choice. In Mate choice. (ed P. Bateson), pp. 109-138. Cambridge University Press, Cambridge.
- Clutton-Brock, T.H. (1989) Mammalian mating systems. Proceedings of the Royal Society of London, series B, 236, 337-372.
- Duncan, A., Baker, G.B., & Montgomery, N. (1999). The action plan for Australian bats. Environment Australia, Canberra.
- Eby, P. (1991) Seasonal movements of grey-headed flying-foxes, Pteropus poliocephalus (Chiroptera: Pteropodidae), from two maternity camps in northern New South Wales. Wildlife Research, 18, 547-549.
- Eby, P. (1995). The biology and management of flying foxes in NSW. Rep. No. 18. N.S.W. National Parks and Wildlife Service, Hurstville.
- Eby, P. (1996) Interactions between the grey-headed flying-fox, Pteropus poliocephalus (Chiroptera: Pteropodidae) and its diet plants - seasonal movements and seed dispersal. Ph.D, University of New England, Armidale.
- Eby, P. (1998) An analysis of diet specialization in frugivorous i (Megachiroptera) in Australian subtropical rainforest. Australian Journal of Ecology, 23, 443-456.
- Eby, P. & Palmer, C. (1991) Flying-foxes in rainforest remnants in northern New South Wales. In Rainforest Remnants. (ed S. Phillips), pp. 48-56. NSW National Parks and Wildlife Service, Lismore.
- Eby, P., Richards, G., Collins, L., & Parry-Jones, K. (1999) The distribution, abundance and vulnerability to population reduction of a nomadic nectarivore, the grey-headed flying-fox, Pteropus poliocephalus in New
- South Wales, during a period of resource concentration. Australian Zoologist, 31, 240-255.
- Emlen, S.T. & Oring, L.W. (1977) Ecology sexual selection and the evolution of mating systems. Science, 197, 215-223.
- Fraser, G.C., Hooper, P.T., Lunt, R.A., Gould, A.R., Gleeson, L.J., Hyatt, A.D., Russell, G.M., & Kattenbelt, J.A. (1996) Encephalitis caused by a Lyssavirus in fruit bats in Australia. Emerging Infectious Diseases, 2, 327-31.
- Fujita, M.S. & Tuttle, M.D. (1991) Flying-foxes (Chiroptera: Pteropodidae): threatened animals of key ecological and economic importance. Conservation Biology, 5, 455-463.
- Hall, L.S. (1987) Identification, distribution and taxonomy of Australian flying-foxes (Chiroptera: Pteropidae). Australian Mammalogy, 10, 75-79.
- Hall, L.S. & Richards, G.C. (2000) Flying foxes: fruit and blossom bats. University of New South Wales Press, Sydney.
- Halpin, K., Young, P.L., Field, H., & Mackenzie, J.S. (1999) Newly discovered viruses of flying foxes. Veterinary microbiology., 68, 83-7.
- Kaiser, D.J.T. (2004) Territoriality in the Grey-Headed Flying-Fox (Pteropus poliocephalus) in New South Wales, Australia. MSc thesis, University of Groningen, Groningen.
- Kokko, H., Rintamaeki, P.T., Alatalo, R.V., Hoeglund, J., Karvonen, E., & Lundberg, A. (1999) Female choice selects for lifetime lekking performance in black grouse males. Proceedings of the Royal Society of London, series B, 2109-2115.
- Lunney, D. & Moon, C. (1997) Flying-foxes and their camps in the remnant rainforests of north-east New South Wales. In "Australia's ever changing forests' III: Proceedings of the third national conference on Australian forest history (ed J. Dargavel), pp. 247-277. Centre for Resource and Environmental Studies, Australian National University, Canberra.
- Lunney, D., G. Richards, and C. Dickman. 2008. Pteropus poliocephalus in Red List of Threatened Species (IUCN, ed.).
- Markus, N. (2002) Behaviour of the black flying fox Pteropus alecto: 2. Territoriality and courtship. Acta Chiropterologica, 4, 153-166.
- Martin, L., Kennedy, J.H., Little, L., & Luckhoff, H.C. (1993) The reproductive biology of Australian flying-foxes (genus Pteropus). In Ecology, evolution and behaviour of bats (ed S.M. Swift), pp. 167-186. Oxford, London.
- Martin, L., Kennedy, J.H., Little, L., Luckhoff, H.C., O'Brien, G.M., Pow, C.S.T., Towers, P.A., Waldon, A.K., & Wang, D.Y. (1995) The reproductive biology of Australian flying-foxes (genus Pteropus). In Symposia of the Zoological Society of London, No 67. pp. 167-184. Clarendon Press, Oxford, UK.
- Martin, L., Towers, P.A., McGuckin, M.A., Little, L., Luckhoff, H., & Blackshaw, A.W. (1987) Reproductive biology of flying foxes (Chiroptera: Pteropodidae). Australian Mammalogy, 10, 115-118.
- McCracken, G.F. & Wilkinson, G.S. (2000) Bat mating systems. In Reproductive biology of bats. (ed P.H. Krutzsch), pp. 321-362. Academic Press, San Diego.
- McWilliam, A.N. (1986) The feeding ecology of Pteropus in northern New South Wales, Australia. Myotis, 23, 201-208.
- Meehl, G.A. & Tebaldi, C. (2004) More Intense, more frequent, and longer lasting heat waves in the 21st century. Science, 305, 994-997.
- Mickleburgh, S.P., Hutson, A.M., & Racey, P.A. (1992) Old world fruit bats: an action plan for their conservation. I. U. C. N/S. S. C., Gland, Switserland.
- Nelson, J.E.W. (1963) The biology of the flying fox (genus Pteropus) in south-eastern Queensland. Ph.D., University of Queensland, Brisbane,.
- Nelson, J.E.W. (1964) Vocal communication in Australian flying foxes (Pteropodidae; Megachiroptera). Zeitschrift für Tierpsychologie, 27, 857-870.
- Nelson, J.E.W. (1965a) Behavior of Australian Pteropodidae (Megachiroptera). Animal Behaviour, 13, 544-557.
- Nelson, J.E.W. (1965b) Movements of Australian flying foxes (Pteropodidae: Megachiroptera). Australian Journal of Zoology, 13, 53-73.
- Neuweiler, G.v. (1968) Verhaltungsbeobachtungen an einer indischen Flughundkolonie (Pteropus giganteus brünneus). Zeitschrift für Tierpsychologie, 26, 166-199.
- O'Brien, G.M. (1993) Seasonal reproduction in flying foxes, reviewed in the context of other tropical mammals. Reproduction Fertility and Development, 5, 499-521.
- Parry-Jones, K. (1987) Pteropus poliocephalus (Chiroptera - Pteropodidae) in New South Wales. Australian Mammalogy, 10, 81-85.
- Parry-Jones, K. & Augee, M.L. (1991) Food Selection by Grey-Headed Flying Foxes (Pteropus poliocephalus) occupying a summer colony site near Gosford, New-South-Wales. Wildlife Research, 111-124.
- Parry-Jones, K.A. & Augee, M.L. (1992) Movements of Grey-Headed Flying Foxes (Pteropus poliocephalus) to and from a Colony Site on the Central Coast of New-South- Wales. Wildlife Research, 19, 331-40.
- Parry-Jones, K.A. & Augee, M.L. (2001) Factors affecting the occupation of a colony site in Sydney, New South Wales by the Grey-headed Flying-fox, Pteropus poliocephalus (Pteropodidae). Austral Ecology, 26, 47-55.
- Pook, A.G. (1977) Breeding the Rodrigues fruit bat. Journal of Jersey Wildlife Preservation Trust, 14, 30-37.
- Pritchard, K. (2001) George (1978-2001). Friends of bats newsletter (Ku-rin-gai Bat Conservation Society), 61, 7.
- Ratcliffe, F. (1931) The flying fox (Pteropus) in Australia. H.J. Green government printer, Melbourne.
- Ratcliffe, F. (1932) Notes on the fruit bats (Pteropus spp.) of Australia. The Journal of Animal Ecology, 1, 32-57.
- Richards, G. (2000). In Proceedings of a workshop to assess the status of the Grey-headed Flying Fox (eds G. Richards & L. Hall). Australasian Bat Society, Canberra.
- Richards, G.C. (1995) Ecological interactions of fruit bats in Australian ecosystems. In Symposia of the Zoological Society of London, No 67. pp. 79-96. Clarendon Press, Oxford, UK.
- Saunders, D.A., Hobbs, R.J., & Margules, C.R. (1990) Biological consequences of ecosystem fragmentation: a review. Conservation Biology, 5, 18-18.
- Spencer, H.J., Palmer, C., & Parry-Jones, K. (1991) Movements of fruit bats in eastern Australia, determined by using radio-tracking. Wildlife Research, 18, 463-468.
- Temminck, C.J. (1825) Vues générales sur l’ordre des cheiroptères. In Monographies de mammalogie, ou description de quelques genres de mammifères, dont les espèces dont été observées dans les différens musées de l’Europe. pp. 157-204. G. Dufour & E. D'Ocagne, Paris.
- Tidemann, C.R. (1999) Biology and management of the grey-headed flying-fox, Pteropus poliocephalus. Acta Chiropterologica, 1, 151-164.
- Tidemann, C.R. (2003) Displacement of a flying fox camp using sound. Ecological Management and Restoration, 4, 5-7.
- Tidemann, C.R. & Vardon, M.J. (1997) Pest, pestilence, pollen and pot-roasts: the need for community based management of flying foxes in Australia. Australian Biologist, 10, 77-83.
- Tidemann, C.R., Vardon, M.J., Loughland, R.A., & Brocklehurst, P.J. (1999) Dry season camps of flying-foxes (Pteropus spp.) in Kakadu World Heritage Area, north Australia. Journal of Zoology, 247, 155-164.
- Tidemann, C.R., Vardon, M.J., Nelson, J., Speare, R., & Gleeson, L. (1997) Health and conservation implications of Australian bat Lyssavirus. Australian Zoologist, 30, 369-376.
- Vardon, M.J. & Tidemann, C.R. (1995) Harvesting of flying-foxes (Pteropus spp.) in Australia: could it promote the conservation of endangered Pacific island species? In Conservation through sustainable use of wildlife (eds G. Grigg, P. Hale & D. Lunney), pp. 82-85, Brisbane, Australia.
- Webb, N.J. & Tidemann, C.R. (1995) Hybridisation between black (Pteropus alecto) and grey-headed (P. poliocephalus) flying-foxes (Megachiroptera: Pteropodidae). Australian Mammalogy, 18, 19-26.
- Welbergen, J.A. (2001). First year report: The social organisation of the Grey-Headed Flying-Fox, Pteropus poliocephalus: colony composition and behaviour. The Department of Zoology; University of Cambridge, Cambridge.
- Welbergen, J.A. (2002). Second year report: The social organisation of the Grey-Headed Flying-Fox,Pteropus poliocephalus: causes, consequences, and conservation. The Department of Zoology, The University of Cambridge, Cambridge.
- Welbergen, J.A. (2005) The social organisation of the grey-headed flying-fox. Ph.D thesis, University of Cambridge, Cambridge.